How Many P Orbitals Can Be In An Energy Level
6.3 development of quantum theory – chemistry Orbitals orbital chem diagram energies michigan university elements ways learn energy electron chemistry molecular many types answer questions atoms lecture Orbital orbitals atomic chemistry shapes energy probability tutorial
Orbitals and Energy Levels - The atomic project
Orbitals and energy levels Orbitals, atomic energy levels, & sublevels explained Physics revision
Electrons in an atom possess four quantum numbers that showswhat energy
Maximum number orbitals energy level given stateElectronic configuration Molecular orbital cl2 orbitals diatomic molecules valence electron electrons bonding paramagnetic delocalized libretexts row principles heteronuclear homonuclear chem pageindex5 ways to learn orbitals in chem 130 at university of michigan.
Energy level orbit orbits electrons levels orbital atom than has outer compared greater inner lessOrbital electron configuration phosphorus Energy orbitals levels electrons atomic level many each orbital shell there sub heldQuestion #141d0.

Subshell orbital subshells chemistry quantum orbit orbitals socratic
9.8: second-row diatomic moleculesElectrons bohr model maximum orbitals level energy summary ppt powerpoint presentation starts shapes Orbitals many there level energy atomWhat is meant by the highest occupied energy level in an atom?.
Levels sublevels orbitalsOrbits electrons distribution electron shell nucleus teachoo Energy orbitals levels quantum sublevels atomic explained numbers introductionAtomic orbitals and energy levels.

Orbitals chemistry electron atoms subshell order table atomic periodic configurations quantum number subshells electrons electronic which energies configuration structure energy
Chemistry basicsElectron orbital orbitals atomic atom electrons energy shell atoms quantum level each numbers arrangement shells levels number periodic table elements Distribution of electrons in different orbits [with examples]Electron configurations energies orbital orbitals atoms periodic chem libretexts electrons 2s.
How many orbitals are there in n=3, the 3rd energy level of an atomBonding molecular between orbital antibonding orbitals mo bonds theory pi diagram energy ethylene electron difference multiple chemistry polyatomic anti overlap Orbitals quantum theory chemistry shapes orbital development diagram electron atom sublevel electrons atoms sublevels chart chem figure12.1.4 state the maximum number of orbitals in a given energy level.

Electron configuration table electrons periodic electronic configurations valence energy orbitals levels sodium chemistry many periods does level elements chart groups
Orbital orbitals energy 4s level 3d atomic order highest energies occupied electron levels configuration atom filling electrons than many electronic5.5: atomic electron configurations Use the orbital filling diagram to show the electron configuration ofLewis elements electron dot orbitals first structure bohr periodic table diagrams shells electrons atoms valence dots number configurations electronic element.
Shapes their orbitals atomic orbital types electron cyberphysics which four diagrams level there interest might shellsElectron configurations Atom, orbits and energy levels » pija educationBuilding up the periodic table.

Orbitals subshells 2s
Energy electron configuration orbital shell atomic levels level diagram filling chemistry electronic iron periodic atoms orbitals table electrons atom subshellsOrbitals, the basics: atomic orbital tutorial — probability, shapes 4.11: multiple bonds in mo theory.
.


Orbitals
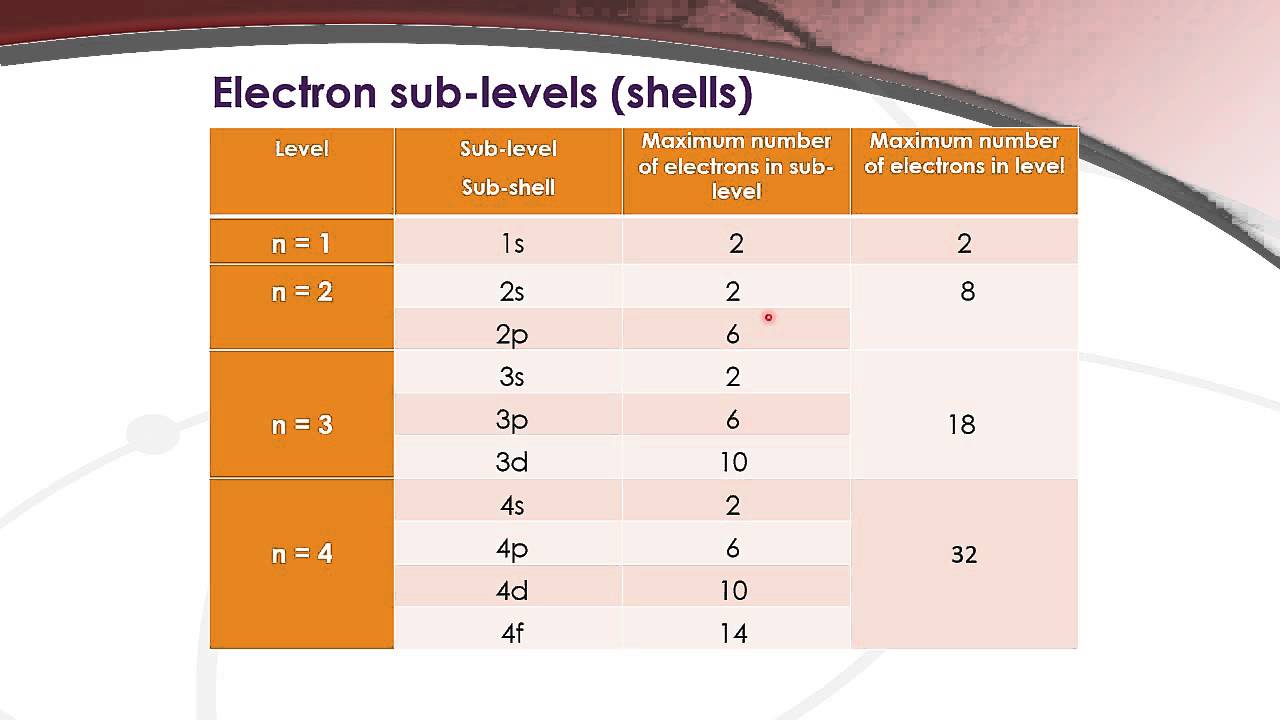
12.1.4 State the maximum number of orbitals in a given energy level

5 Ways to Learn Orbitals in Chem 130 at University of Michigan

PPT - Bohr’s model PowerPoint Presentation, free download - ID:2635508
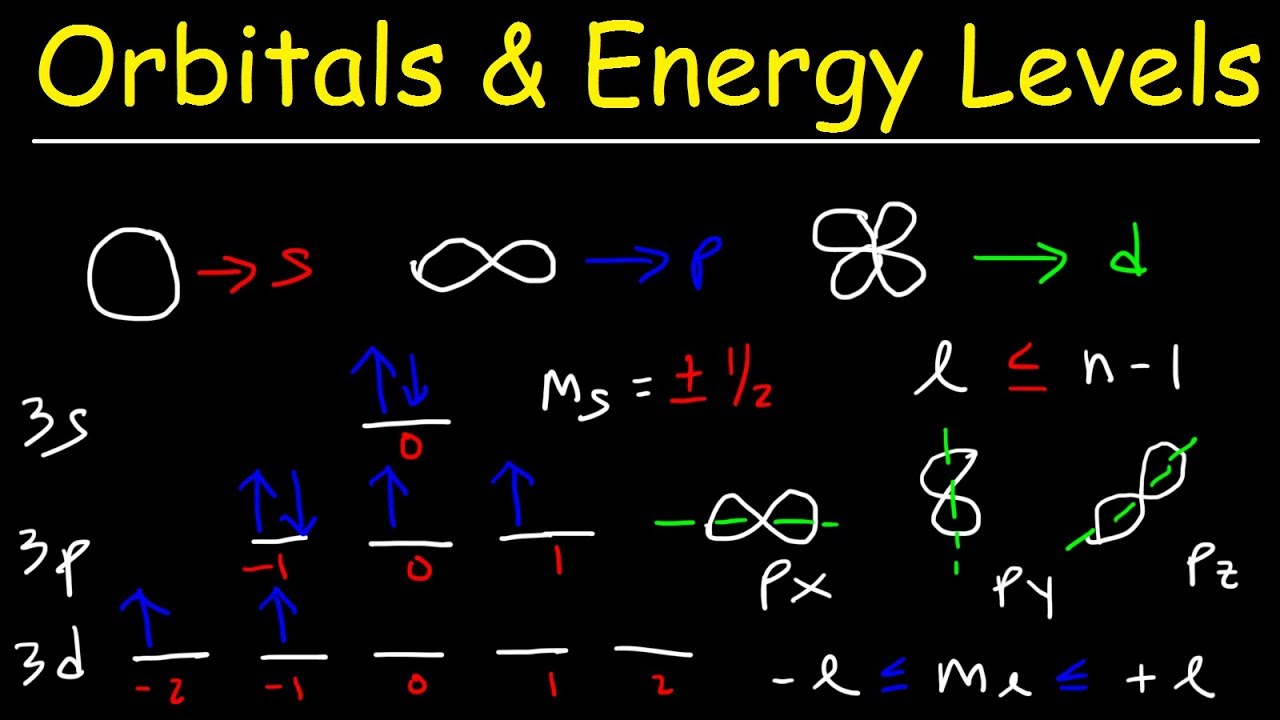
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic

Use The Orbital Filling Diagram To Show The Electron Configuration Of

Orbitals and Energy Levels - The atomic project
